Overview:
Process control systems, sometimes called industrial controls systems (ICS), function as pieces of equipment along the production line that test the process in a variety of ways and return data for monitoring and troubleshooting.
We’ve studied process control systems in detail in class. Based on my research of the industrial application of process control systems, I was able to identify the four vital elements of industrial process control systems: a measurement that indicates the current status of the condition of the process, a controller to take action based on that measurement and a set value, an output signal to manipulate the process that results from the controller, and the process in its entirety that reacts to the signal (input or output).
Distributed Control Systems are specially designed systems to control complex and geographically distributed processes. These controllers are distributed in the entire plant area; discrete field devices such as actuators and sensors are connected to these controllers and also maintain continuous communication with operating PCs through a high-speed communication network.
The basic element of the DCS system is an engineered PC controlling the distributed controllers, which then control connected field devices. An operating station is used to monitor the field parameters graphically and to log the data (and communication media to establish data transfer between the controllers and the operating stations). DCS facilitates the human machine interface, trend display and face plates for the effective monitoring of industrial processes.
Chosen System: PlantPAx Distributed Control System
Overview: The PlantPAx system uses a common automation platform for integration between the critical areas of a plant. The modern DCS connects process, discrete, power, information, and safety control into one plant-wide infrastructure.
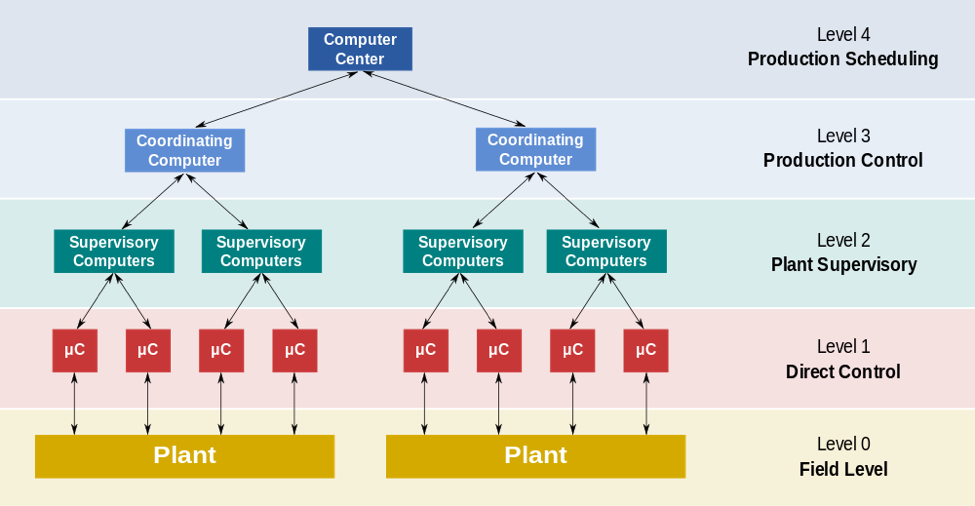
The key “levels” of control of the PlantPAx are as follows:
- Level 0 constitutes the field devices: these are the devices that measure the flow or temperature of the plant, and final control elements like control valves.
- Level 1 contains the industrialized input/output modules and the distributed electronic processors associated with these modules.
- Level 2 contains the supervisory computers which collect information from processor nodes on the systems, and provide the operator control screens.
- Level 3 is the production level which simply monitors the production and targets.
- Level 4 is the production scheduling level.
Level 1 and 2 are the functional levels of traditional DCS, where all equipment is part of an integrated system from a single manufacturer. The processor nodes and operator graphical displays are connected over proprietary or industry standard networks. Processors receive information from input modules and then process the information and decide control actions to be signaled by the output modules. The field inputs and outputs can be analog signals or two state signals that simply switch on and off. The DCS is connected to the sensors, and uses the methods of set-point control that we have studied to control the flow of material through the plant. Most of the systems in chemical plants use PID controllers: the PID controller is fed by a flow meter using a control valve as the final control element. The DCS will then send the set-point required by the process to the PID controller which signals the valve to operate so that the process achieves the set-point. Most large chemical plants have several thousand input and output points and thus utilize large DCS. This semester, we studied the transfer functions that describe PID control: the equation includes terms for proportional, integral, and derivative control, but I appreciated learning about DCS systems because we can see the larger scale application of these control systems in an integrated plant. In the temperature lab, for example, we observed how proportional control on its own is an inefficient means of controlling processes due to steady-state offset, and we saw how playing with the integral and derivative terms of a PID controller can reduce off-set and minimize oscillations. Essentially, we learned that PID is typically the most effect means of controlling a system. However, the information I learned while compiling this profile on the DCS system illustrated the application of the PID controller in large-scale chemical plant.
References: